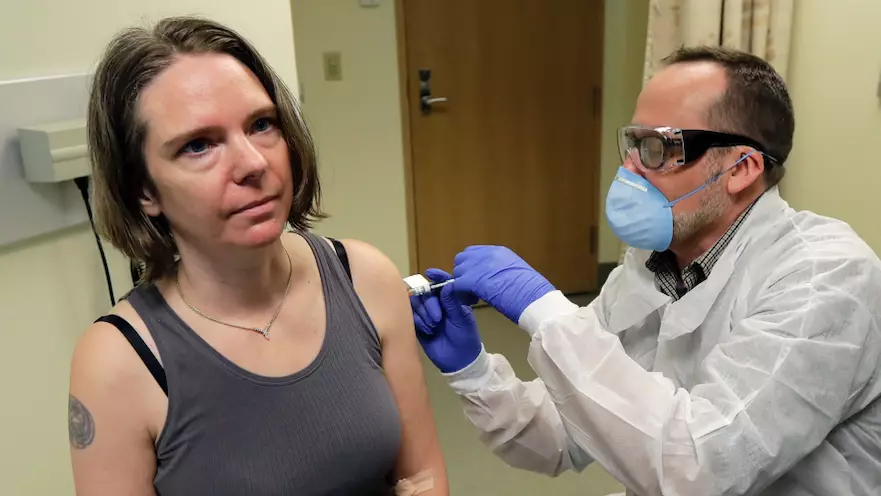
A mum from Seattle was one of the first people to trial an experimental coronavirus vaccine yesterday.
US researchers started the human trial on Monday, administering a shot to several carefully selected volunteers, including 43-year-old mum-of-three Jennifer Haller.
Advert
As she was injected with the vaccine, Jennifer said her kids, Hayden and Ellie, think that it's "cool" she's taking part in the trial.
The early-stage tests are currently being led by Dr Lisa Jackson, a senior investigator at Kaiser Permanente Washington Health Research Institute in Seattle, Washington - which has seen 42 of America's 69 deaths.

The study asked healthy people aged between 18 and 55 to volunteer, and carefully hand-picked the best suited for the trial.
Advert
Over the next six weeks, the experiment will enroll 45 participants in total - including Jennifer - and they will all be given two doses, a month apart.
It will work by giving some individuals higher doses than others in order to test how strong the inoculations might need to be.
Researchers will then be monitoring carefully for any side-effects and drawing blood samples in order to see whether or not the vaccine is aiding the immune system.

Advert
The ultimate aim is not only to test the safety of the vaccine, but also its ability to make volunteers immune from COVID-19.
Jennifer said she was "feeling great" as she left the examination room after being injected.
Speaking about why she got involved with the study, she said: "We all feel so helpless. This is an amazing opportunity for me to do something."
Study leader Dr Jackson said: "Everyone wants to do what they can in this emergency. We're team coronavirus now".
Advert

She added: "We don't know whether this vaccine will induce an immune response, or whether it will be safe. That´s why we´re doing a trial.
"It's not at the stage where it would be possible or prudent to give it to the general population.
"This work is critical to national efforts to respond to the threat of this emerging virus."
Advert
The National Institute of Allergy and Infectious Diseases allowed the new vaccine to be fast-tracked into clinical trials.
This is the first of three test stages that the vaccine must be put through and pass before it will be deemed safe to use.

Director Anthony S. Fauci said in a statement: "Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority."
He added: "This Phase 1 study, launched in record speed, is an important first step toward achieving that goal."
It's not the only potential vaccine in the pipeline. In fact, dozens of research groups globally are racing to create a vaccine to protect us against COVID-19.
Inovio Pharmaceuticals is another group following closely behind the Seattle team.
All going well, they are expected to begin safety studies of their own next month across the U.S., China and South Korea.
Featured Image Credit:Topics: Science, Coronavirus, Health, Covid-19